Soil pH
Soil acidity reflects the concentration of hydrogen ions (H+), which are measured as pH units with a range from 0 (very acid) to 14 (very basic), with pH7 being neutral. Each unit change represents a 10-fold difference in acidity. For example, pH5 is 10-fold more acidic than pH6.
Soil pH affects plant growth by altering the activity of soil microorganisms and the availability of nutrients and toxic metals. While some plants require an acid soil, most prefer a pH in the range 5.8–6.5.
Problems
Because our native soils are acidic, problems associated with acid soils can occur here. Problems associated with alkaline soils can occur if too much lime has been applied to the soil.
| Very acid soils | Alkaline soils |
|---|---|
| Aluminum toxicity to plant roots* | Iron deficiency |
| Manganese toxicity to plants | Manganese deficiency |
| Calcium & magnesium deficiency | Zinc deficiency |
| Molybdenum deficiency in legumes | Excess salts (in some soils) |
| Phosphate tied up by iron and aluminum | Phosphate tied up by calcium and magnesium |
| Poor bacterial growth | Bacterial diseases in potatoes |
| Reduced nitrogen transformations |
* In a mineral soil with a pH below 6 or an organic soil with a pH below 5, metallic micronutrients like copper, iron, manganese, zinc, and aluminum are available for plant uptake because they can dissolve in soil water. Because aluminum is abundant in silt and clay, aluminum toxicity is the major problem with our acid soils.
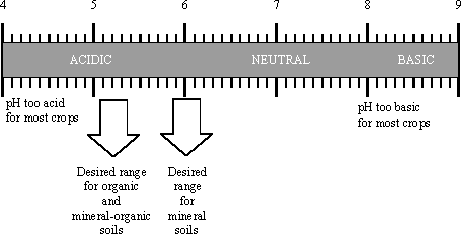
ideal range
The ideal pH range depends both on the plant species and on how much organic matter is present in the soil:
- Mineral soils: Organic matter comprises ‹ 20%.
- Organic soils: Organic matter comprises › 20%.
 NCDA&CS Agronomic Services
NCDA&CS Agronomic Services
Learn about soil organic matter.
nutrient availability
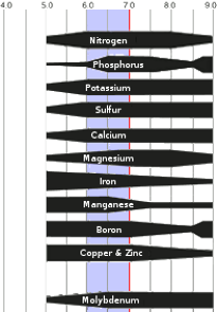
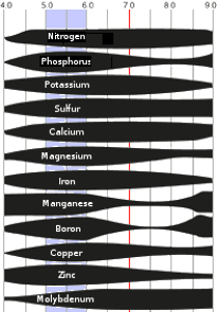
Bar thickness is proportional to nutrient availability:
Mineral Soils
The range of greatest nutrient availability is pH 6–7.
Organic Soils
The range of greatest nutrient availability is pH 5–6.
How to modify acidity
Soil Acidity and Liming
Lowering Soil pH
Soils in the Piedmont tend to be acidic (pH 4.8–5.3) and low in organic matter (‘mineral soils’). The pH of these soils needs to be raised by approximately 1 unit to be optimal for most plants. Note that fill dirt may not be representative of native soil.
To decrease soil acidity (raise pH)
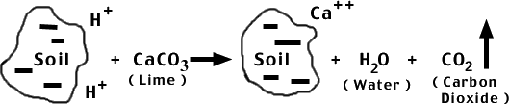
Lime decreases soil acidity by changing some of the soil hydrogen ions into water and carbon dioxide. In the reverse process, basic cations (Ca2+, Mg2+, K+) are lost through crop uptake or leaching from rainfall/irrigation and replaced by H+.
 SC CES Soil Acidity and Liming - Part 2
SC CES Soil Acidity and Liming - Part 2
While the main function of lime is to increase soil pH, lime also adds calcium and magnesium. The most common liming materials are agricultural limestones.
- Calcitic limestone is mostly calcium carbonate (CaCO3).
- Dolomitic limestone is a mixture of calcium and magnesium carbonates. NC law requires a minimum of 6% soluble magnesium for limestone to be labeled dolomitic.
Amount to apply
The amount of lime needed to correct soil acidity depends on soil composition. Because lime chemistry is complex, it is impossible to know how much is needed unless you have the soil tested. Several factors affect the amount of lime needed:
- current soil pH
- soil buffering capacity
- soil cation exchange capacity (CEC), which is the quantity of exchangeable aluminum and hydrogen ions.
Clay and organic matter have both high cation exchange capacity and high buffering capacity. Soils rich in either require greater amounts of lime to change the pH.
tips
- Test your soil first
Because lime is poorly soluble, it works slowly and special care is needed to ensure good results. It is best to take soil samples before applying lime because the soil test cannot detect the latent liming power from a previous application. If your soil has been limed previously, be sure to indicate this on the soil test form so that the NCDA factors it into the lime recommendation. Otherwise you will overcorrect your soil pH.
Agronomic Services — Soil Test Forms & Info - A fine grind gives better results
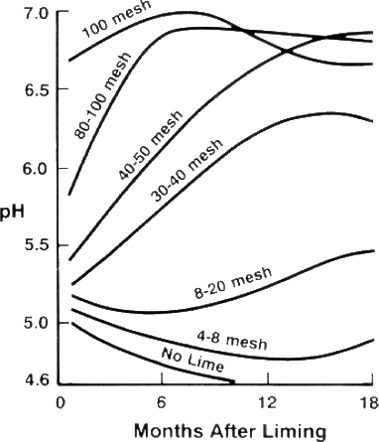
Because lime neutralizes acidity only in a small area around the particle, the finer the grind (the larger the mesh size), the faster and more even the neutralizing effect. Although prilled or granular limestone products are easier to spread, pH change will occur less evenly. - Mix into the bed well in advance of planting.
Because lime moves through the soil very slowly (less than 1 inch per year) it should be incorporated evenly to the entire depth of the bed, preferably about 6 months prior to planting.
 NC CES SoilFacts: Soil acidity and liming
NC CES SoilFacts: Soil acidity and liming
To increase soil acidity (lower pH)
Specialty fertilizers for acid-loving plants are the best choice. Most contain ammonium sulfate or sulfur-coated urea, which both provide nitrogen and decrease pH.
Lowering Soil pH
Factors affecting soil pH
Much of the content below is adapted from Soil Acidity and Liming - Part 2.
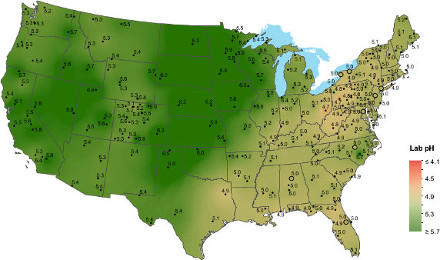
Geographic region
Geographic regions have characteristic pH ranges because the five soil-forming factors (parent material, climate, living organisms, topography, and time) are regional characteristics.
- Soils in the Piedmont are acid because the granite rocks that formed them are acidic.
- Soils formed under high rainfall conditions (eastern USA) are more acid than those formed under dry conditions (western USA). As water passes through soil, it leaches basic cations like calcium, magnesium, and potassium into drainage water. The basic cations are then replaced by acidic cations like aluminum and hydrogen.
 National Atmospheric Deposition Program National Trends Network
National Atmospheric Deposition Program National Trends Network
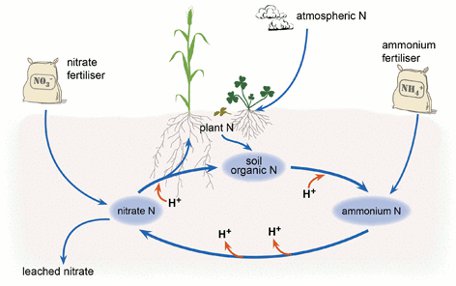
Fertilizers
Bacterial conversion of fertilizer nitrogen into nitrate releases H+ and thus increases soil acidity. This is true for both chemical fertilizers, which contain ammonia or urea, and organic fertilizers, which contain amino acids.
Plant uptake
Plants take up basic cations like potassium, calcium, and magnesium. When these are removed from the soil, they are replaced with H+ in order to maintain electrical neutrality.
Dissociation of carbonic acid
Carbonic acid forms readily in soils when CO2 and water are present. It then dissociates, releasing hydrogen ions in the soil water.
H2CO3- → H+ + HCO3-
Acid rain, sulfur-containing fertilizers
Coal-fired power plants release sulfur to the atmosphere, where it is converted into sulfuric acid and falls on the soil as acid rain, sleet, or snow.
| pH | Soil Condition |
|---|---|
| <4.0 | soil contains free acids probably as a result of sulfide oxidation |
| <5.5 | soil exchange complex is dominated by aluminum |
| <7.8 | soil pH is controlled by a range of factors |
| >7.8 | soil contains calcium carbonate |
